Aptar is seeking Emergency Use Authorization (EUA) from the German Federal Institute for Drugs and Medical Devices (BfArM) for a solution that allows for easy disinfecting of FFP2 filtering facepiece respirators (FFP2 masks). The FFP2 masks are needed by healthcare personnel due to the global shortage of disposable masks during the COVID-19 pandemic.
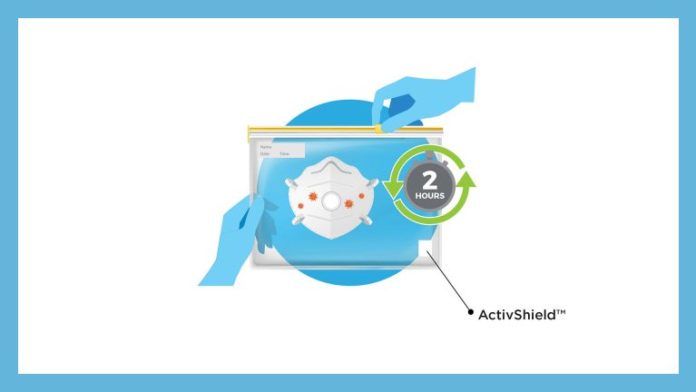
In this simple disinfecting process, the FFP2 mask and a small strip of Aptar’s ActivShield technology are placed inside any commonly available one-gallon plastic bag. The strip releases a controlled amount of chlorine dioxide inside the sealed bag to decontaminate the FFP2 mask. The process takes only two hours until the mask is ready to wear again. It can be performed on-site at the local hospital where the mask is being used. The EUA submission in Germany is part of Aptar’s strategy to expand global access to FFP2 respirators allowing for the decontamination and reuse of masks for ten cycles. This innovative patent pending technology can efficiently and effectively disinfect FFP2 masks used by healthcare workers and first responders.
The German EUA BfArM submission follows Aptar’s US FDA Emergency Use Authorization submission in early April. Germany is thus the first country in the EU where an application to this effect has been submitted to the authorities.
“We are proud to continue to support the fight against the COVID-19 pandemic by furthering our efforts to bring this innovative technology to the global health care community. We have a long-standing presence in Germany, and we will continue to support the communities where we live and work,” said Stephan Tanda, Aptar president and chief executive officer. “Our technology provides a unique, simple, and effective way to help solve the critical problem of PPE shortages we’re currently facing. We look forward to working with the US FDA and BfArM to bring ActivShield to market and fulfill the ongoing unmet need.”
Chlorine dioxide has been widely used as a disinfectant in different industries, including the paper industry, drinking water treatment, food processing, and medical equipment. Aptar’s delivery mechanism uses the disinfecting properties of chlorine dioxide in a controlled, sustained release within a contained volume.
Studies on the ActivShield system show the treatment to be 99.999% effective against viruses. Detailed data on the effectiveness and safety of the ActivShield system is available at www.aptaractivshield.com.








