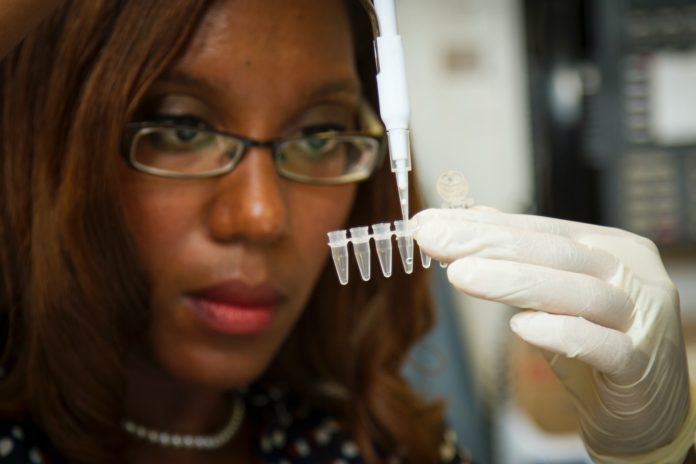
aTyr Pharma, a biotherapeutics company engaged in the discovery and development of innovative medicines based on novel immunological pathways, announced that it had dosed the first patient in a Phase 2 study evaluating its lead therapeutic candidate, ATYR1923, in Covid-19 patients with severe respiratory complications. The study is expected to enroll 30 patients at up to 10 centers in the US, and the company expects to have the majority of centers enrolling within the coming weeks.
ATYR1923 is a potential first-in-class immunomodulator that has been shown preclinically to downregulate T-cell responses and improve inflammation and lung function. ATYR1923 is currently being evaluated in a Phase 1b/2a multi-center trial for patients with pulmonary sarcoidosis, a severe inflammatory lung disease. There is strong scientific rationale for the hypothesis that ATYR1923 may help regulate the excessive inflammatory response in the lungs, primarily driven by T-cells, seen in many Covid-19 patients.
“As patients continue to be hospitalized due to Covid-19 throughout the US, there is a need for effective therapies to treat severe inflammation associated with this disease. ATYR1923 is a novel therapeutic candidate specifically designed to address aberrant immune response and inflammation in the lung,” said Sanjay Shukla, president, and chief executive officer of aTyr. “This is an important step forward to assess the potential utility of ATYR1923 in this subset of Cocid-19 patients who experience serious respiratory complications that can lead to longer hospitalization stays and, in some cases, they need mechanical ventilation and intensive care treatment.”
The Phase 2 clinical trial is a randomized, double-blind, placebo-controlled study of ATYR1923 in hospitalized Covid-19 positive patients with severe respiratory complications who do not require mechanical ventilation. Patients enrolled in the trial will be assigned to one of three cohorts of 10 patients each. Patients will receive a single intravenous (IV) dose of either 1.0 or 3.0 mg/kg ATYR1923 or placebo. Patients will be followed for 60 days post-treatment. The trial is designed to evaluate the preliminary safety and efficacy of ATYR1923 compared to placebo. This is done by assessing key clinical outcome measures such as fever and hypoxia as well as inflammatory biomarkers.
“We are encouraged by the significant interest that we have received from respiratory specialists throughout the country wanting to participate in this trial. We have currently initiated five sites and expect the majority of sites to be active within the coming weeks. We look forward to completing this important study and report data from this trial later this year,” said Shukla.