Glenmark Pharmaceuticals, a research-led, global integrated pharmaceutical company, recently launched Nintedanib 100 and 150 mg capsules to treat pulmonary fibrosis in India. Glenmark, being a leader in the respiratory area, has been amongst the first to launch the branded generic version at an affordable cost for the treatment of Pulmonary Fibrosis in India. This will provide patients a far more cost-effective treatment option and enable doctors to treat a wider patient population in the country.
Nintedanib is approved by the Indian drug regulator to treat Idiopathic (unknown cause) Pulmonary Fibrosis (IPF). Since IPF is a progressive disease that gets worse over time, starting treatment early and continuing treatment are essential to slow disease progression. Therefore, a lower monthly treatment cost becomes crucial to ensure patients adhere to prescribed treatment in the long term.
So far, Nintedanib has been studied extensively in various controlled clinical trials that have established its efficacy and safety. In a recently published Inbuild trial, Nintedanib showed a significantly lower annual rate of decline in FVC (Forced Vital Capacity) – a measure of lung health – with various progressive fibrosing interstitial lung diseases4. Moreover, two clinical trials are being rolled out to study Nintedanib’s efficacy, and safety as a treatment of SARS-COV2 induced pulmonary fibrosis in Moderate to Severe Covid-19 patients.
“With limited treatment options available, interstitial lung diseases pose a significant treatment challenge in India. The high price of newer treatments and the pill burden of existing options only add to poor patient adherence. By introducing Nindanib, we hope to substantially reduce both the pill and cost burden for patients in India,” said Alok Malik, group vice president, and business head, India Formulations. He added, “Glenmark continues to invent and innovate healthcare solutions that meet specific and often hard-to-address needs of patients in India and the world.”
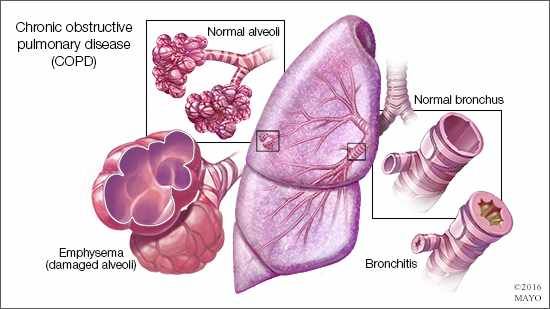
Pulmonary fibrosis (PF) is a respiratory condition characterized by the thickening and scarring of the lungs. This makes it difficult for oxygen to pass through the air sacs and into the bloodstream, causing symptoms such as shortness of breath and dry cough. The average survival rate in patients with IPF is poor, with only 20 to 30% of people surviving at least five years after diagnosis. The most frequent cause of death is respiratory failure. IPF typically affects men over the age of 65 in India, and most people with IPF live only three to five years post-diagnosis if left untreated.