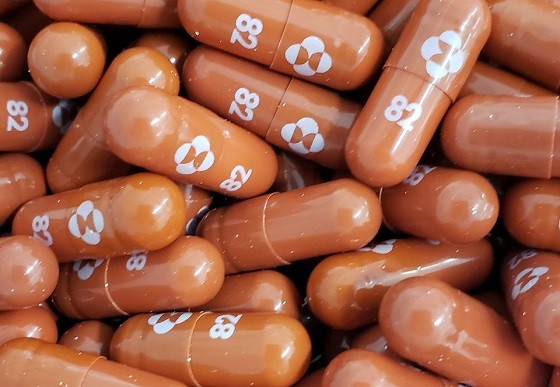
Pharmaceutical giant Merck recently reported positive results on clinical trials for its antiviral medication Molnupiravir against the novel Coronavirus. According to the clinical trial results, patients infected with the mild or moderate form of Covid-19 disease could halve their chances of premature death and/or hospitalization upon treatment with the magic pill.
Molnupiravir antiviral pill is touted as the first oral medicine effective in the treatment of the novel Coronavirus. Molnupiravir is an orally administered drug that blocks the replication of many RNA viruses, including SARS-CoV-2. The drug works by introducing errors into the genetic code of the highly contagious virus.
Shares soar for associated Indian manufacturers
In our previous article dated 29 June 2021, we reported that five Indian pharmaceutical companies, namely, Cipla, Dr Reddy’s Laboratories, Emcure Pharmaceuticals, Sun Pharmaceutical Industries, and Torrent Pharmaceuticals, collaborated for clinical trial assessment of oral dosage form of antiviral Molnupiravir on the treatment of mild Covid-19 OPD cases in the country.
Between March and April 2021, these five pharmaceutical giants entered into a separate licensing agreement with Merck that granted them to manufacture and supply Molnupiravir in India along with over 100 low- and middle-income countries (LMICs). These companies conducted clinical trials on 1,200 mild Covid-19 cases between June and September.
The successful completion of the trials of individual pharma companies would then make them eligible for Molnupiravir production and supply after the go-ahead from regulatory authorities. At least three of the companies are eyeing to seek approval for Molnupiravir by the drug regulator this month following the positive outcome from its clinical trials. Non-exclusive voluntary licensing agreements had also been granted to Aurobindo Pharma, Hetero Labs, and Viatris.
India-based Divi Laboratories, an authorized API (active pharmaceutical ingredient) manufacturer, recorded a 10% record increase in the Nifty Pharma Index, taking its shares to Rs 5,315 per share on BSE on 4 October. MSN laboratories, Honour Lab, Maithri Laboratories, and Optimus Pharma are the other companies involved in manufacturing APIs for Molnupiravir.
After the trial results announcement, shares of Torrent Pharmaceuticals, Sun Pharma, Cipla, and Dr Reddy’s Laboratories recorded a positive 1.2% increase.
Merck aiming for EUA from US FDA
Following positive results from clinical trials of Molnupiravir for treatment of Covid-19 pandemic, the company aims to approach the US Food and Drug Administration (FDA) for Emergency Use Authorization (EUA) of the antiviral medication. Merck also aims to approach other pharmaceutical regulatory bodies for similar use authorization.
Merck is targeting a production of 10 million courses by the end of 2021. The US government has agreed to purchase 1.7 million oral doses of Merck’s wonder drug. The company is also in negotiations with the Malaysian government regarding the procurement of Molnupiravir.