SEA Vision USA will attend the Interphex trade show in New York from 25 to 27 April 2023. This edition of Interphex is particularly important for SEA Vision Group which, at stand 2705, will present its AI-powered Automated Line Clearance software for the first time in the US, installed on a machine developed by Marchesini Group.
The AI-powered Automated Line Clearance – developed by SEA Vision Group together with ARGO Vision, one of the most important Italian companies for computer vision and machine learning – will be shown for the first time in the US, which together with that of the European Union, is one of the most advanced markets in the world in terms of production cycle safety requirements. The union between technologies developed in Italy and their declination for the logic of the American market means that the one presented at Interphex is an extremely refined software, developed using Artificial Intelligence algorithms.
According to first-rate exponents of American entrepreneurship, 2023 will be the year in which AI will make its official entry into large-scale production cycles. SEA Vision USA is ready for this appointment, thanks to over 20 years of expertise in the artificial vision sector, today more than ever hand in hand with the very rapid evolutions linked to the development of AI in the production processes of American pharma market companies.
Smart Line Clearance: safer and more efficient, with AI algorithms
Everybody knows that the line clearance processes are crucial stages performed before production begins. The objective is to ensure that equipment and work areas are clean/free from any residual materials or documents. However, these very important phases still require the manual intervention of operators to perform specific paper-based tasks and activities with consequent risks related to human error.
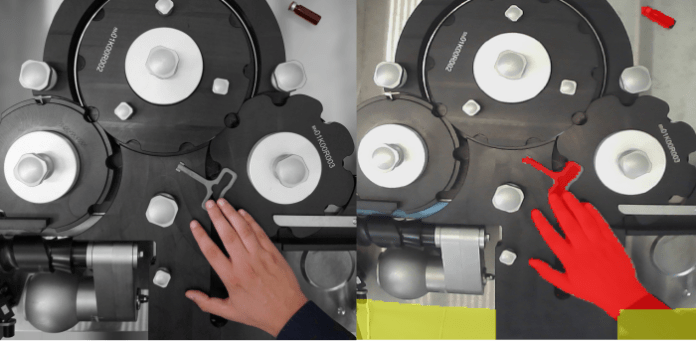
The need for greater safety and smoother processes is the main driver of SEA Vision Group’s Smart Clearance technology. This brand-new solution — already launched at Achema on August 2022 – is driven by AI algorithms to automate the line clearance procedures while avoiding errors, reducing the time required, and boosting the OEE of production lines.
Part of the Industry 4.0 software suite yudoo, Smart Clearance Technology is capable of performing automatic and accurate inspection of the packaging line working areas using cameras and sensors.
These devices are all managed by innovative smart acquisition systems powered by AI algorithms, which analyze the inspected scenes in depth and in real-time, immediately identifying any machine parts out of position, foreign objects, or products passing through the machine. The result is that the clearing and cleaning phases are digitized, and at the same time the checking phase is automated.
The AI system is also able to overcome the limitations of traditional vision systems in terms of glare, shade, or differing light conditions. The advantages are many: more accurate inspections in real time, shorter changeover times, added safety for the business’s quality processes, and enablement of Industry 4.0 factory digitization.
A set of vision controls for vials and syringes
At SEA Vision USA Booth, it will be showing a wide range of controls on a demo station, including a preassembled combination of hardware and software required to perform vision inspection controls on injectables.
These kits (ChecKIT360 and ChecKITassembly) can be installed on any model of vial or syringe filling and capping machine, as well as on syringe assembly machines, to perform different checks and inspections. At the booth it will be possible to see how the software actually works, for example performing the following inspections:
*Vial crimping defects
*Flip-off presence and colors
*Stopper presence and position
*Cap presence and position
*Correct syringe assembly
*Syringe label presence